- It is the main source of energy of our cell.
- Takes place in Mitochondria.
- Movement of protons through inner mitochondrial membrane leads to ATP production
DEFINITION
Oxidative phosphorylation includes the coupling of the oxidation of NADH or FADH2 by the respiratory chain with the synthesis of ATP via gradient of protons across the inner mitochondrial membrane.
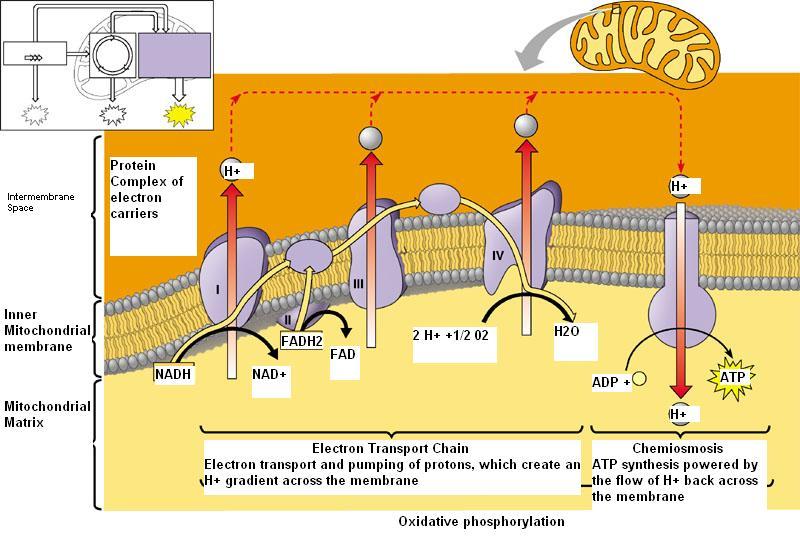
An electron transport chain consists of a properly arranged & oriented set of electron carriers transporting electrons in a specific sequence from a reduced nicotinamide coenzyme (NADH) or a reduced flavin prosthetic group (FADH2) to molecular O2.
Transport chain called the mitochondrial respiratory chain, which forms the final path for electron flow from tissue substrates to molecular O2.
At each step, electrons flow from the reluctant of a redox couple, having a lower redox potential to the oxidant of another redox couple possessing a higher redox potential.
The free energy, liberated during the transmitting of electrons along the chain, is used in forming high-energy bonds of ATP.
DEFINITION
The sequence of reactions whereby the reduced forms of the coenzymes are reoxidized by molecular O2 known as electron transport chain
The chain has principle electron carriers
- NADH dehydrogenase.
- Succinate dehydrogenase.
- Coenzyme Q.
- Cytochromes b2, bH, b560, c1, c, a, & a3 and iron sulphur proteins.
Each of them functions as a redox system with its prosthetic group or metal ions changing alternatively to reductant and oxidant – forms during electron transport.
STEPS OF REACTIONS
To administrate electron transport chain in mitochondria inner membrane four complex presents separately.
Complex I or NADH-CoQ reductase
It transfers reducing equivalents (I.e. H+ and e) from NADH to CoQ through its FMN and iron-sulfur clusters, oxidizing NADH and reducing CoQ.
Complex II or succinate-CoQ reductase
It transports clusters directly to CoQ from succinate through its FAD, cytochrome v560 and iron-sulfur clusters
Complex III or QH2 cytochrome C reductase
- Complex III transfers electrons from QH2 to cytochrome C.
- QH2 transfers one of its two electrons to the Fe2S2
- The second electron is carried successively by cytochrome b2, cytochrome pH and CoQ again.
- Later less received electron passes directly to cytochrome C1.
- Cytochrome C1 then donates the electron to cytochrome C
Complex IV or cytochrome C oxidase
- It transfers one electron from each of four consecutive.
- Ferrocytochrome C molecules to O2 molecules producing four ferricytochrome C molecules and two water molecules.


Leave a Reply
You must be logged in to post a comment.