If a dilute solution is separated from a concentrated solution by a partially permeable membrane, water diffuses across the membrane from the dilute to the concentrated solution. This is known as osmosis.
A partially permeable membrane is porous but allows water to pass through more rapidly than dissolved substances.
Since a dilute solution contains, in effect, more water molecules than a concentrated solution, there is a diffusion gradient which favours the passage of water from the dilute solution to the concentrated solution.
In living cells, the cell membrane is partially permeable and the cytoplasm and vacuole (in plant cells) contain dissolved substances. As a consequence, water tends to diffuse into cells by osmosis if they are surrounded by a weak solution, e.g. freshwater. If the cells are surrounded by a stronger solution, e.g. seawater, the cells may lose water by osmosis. These effects are described more fully later.
Animal cells
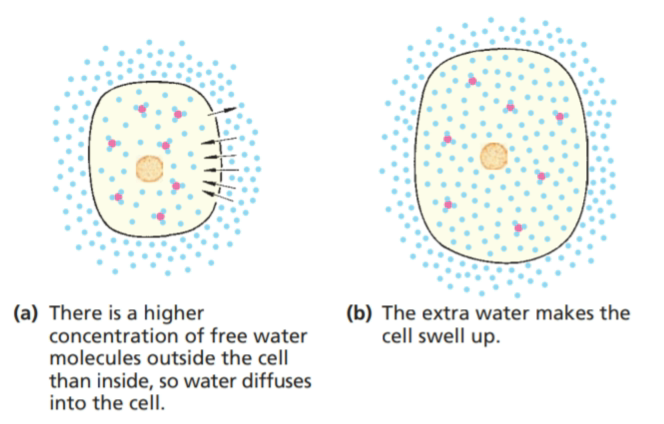
In 3.9 an animal cell is shown very simply. The coloured circles represent molecules in the cytoplasm. They may be sugar, salt or protein molecules. The blue circles represent water molecules.
The cell is shown surrounded by pure water. Nothing is dissolved in the water; it has 100% concentration of water molecules. So the concentration of free water molecules outside the cell is greater than that inside and, therefore, water will diffuse into the cell by osmosis.
The membrane allows water to go through either way. So in our example, water can move into or out of the cell.
The cell membrane is partially permeable to most of the substances dissolved in the cytoplasm. So although the concentration of these substances inside may be high, they cannot diffuse freely out of the cell.
The water molecules move into and out of the cell, but because there are more of them on the outside, they will move in faster than they move out. The liquid outside the cell does not have to be 100% pure water. As long as the concentration of water outside is higher than that inside, water will diffuse in by osmosis.
Water entering the cell will make it swell up and, unless the extra water is expelled in some way, the cell will burst.
Conversely, if the cells are surrounded by a solution which is more concentrated than the cytoplasm, water will pass out of the cell by osmosis and the cell will shrink. Excessive uptake or loss of water by osmosis may damage cells.
For this reason, it is very important that the cells in an animal’s body are surrounded by a liquid which has the same concentration as the liquid inside the cells. The liquid outside the cells is called tissue fluid. and its concentration depends on the concentration of the blood. In vertebrates, the concentration of the blood is monitored by the brain and adjusted by the kidneys.
By keeping the blood concentration within narrow limits, the concentration of the tissue fluid remains more or less constant (see ‘Homeostasis’) and the cells are not bloated by taking in too much water or dehydrated by losing too much.
Plant cell
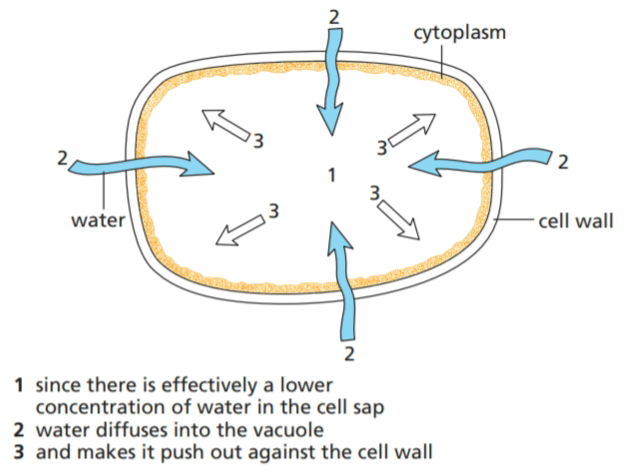
The cytoplasm of a plant cell and the cell sap in its vacuole contains salts, sugars and proteins which effectively reduce the concentration of free water molecules inside the cell. The cell wall is freely permeable to water and dissolved substances but the cell membrane of the cytoplasm is partially permeable. If a plant cell is surrounded by water or a solution more dilute than its contents, water will pass into the vacuole by osmosis. The vacuole will expand and press outwards on the cytoplasm and cell wall. The cell wall of a mature plant cell cannot be stretched, so there comes a time when the inflow of water is resisted by the inelastic cell wall, as shown in Figure.
This has a similar effect to inflating a soft bicycle tyre. The tyre represents the firm cell wall, the floppy inner tube is like the cytoplasm and the air inside corresponds to the vacuole. If enough air is pumped in, it pushes the inner tube against the tyre and makes the tyre hard.
When plant cells have absorbed a maximum amount of water by osmosis, they become very rigid, due to the pressure of water pressing outwards on the cell wall. The end result is that the stems and leaves are supported. If the cells lose water there is no longer any water pressure pressing outwards against the cell walls and the stems and leaves are no longer supported. At this point, the plant becomes limp and wilts




Leave a Reply
You must be logged in to post a comment.