Carbohydrates are classified into two types on the basis of molecular weight.
Micromolecules – Monosaccharides and Oligosaccharides (Including Disaccharides)
Macromolecules – Polysaccharides
The micromolecules have the molecular weight of < 1000 Daltons whereas the
macromolecules have > 1000 Daltons as molecular weight.
- Why do we need carbohydrates in our food? Carbohydrates provides about 50-70% of total energy. We need average carbohydrate requirement in an adult is ~ 400g per day.
- Essential elements in the constitution of carbohydrate – C. H. O
- General Formula – *Cx (H2O)y (Containing more than one-OH groups). The polyhydroxy aldehydes or ketones can also be called as saccharides
- 80% of the dry weight of the plant is carbohydrate.
There are three types of carbohydrates:
- Monosaccharides
- Oligosaccharides
- Polysaccharides
Monosaccharides
They are simplest carbohydrates, with 3 to 7 carbon atoms. All are reducing sugars with a free aldehyde (– CHQ) or ketone (– CO) groups.
- 3-carbons – TRIOSES (C3H6O3) – e.g. Glyceraldehyde (aldose) and Dehydroxyacetone (ketose) (Acetic acid (CH3COOH/ C2H4O2)and Lactic acid are not considered as carbohydrates)
- 4-carbons – TETROSES (C4H8O4) – e.g. Erythrose – an aldose (forms raw-material forlignin)
- 5-carbons – PENTOSES (C5H10O5) – e.g. Ribose (present in RNA, ATP and vitamin B2), Xylose and Arabinose – all aldoses, Ribulose – a ketose.
- 6-carbons – HEXOSES (C6H12O6) – e.g. Glucose, Galactose, Mannose (All aldose sugars) and Fructose (Ketose-sugar). Alcohol of mannose, called Mannitol, is found in brown algae.
- 7-carbons – HEPTOSES (C7H14O7) – e.g. Glucoheptose (Both Pentoses and Hexoses may occur in Ring form and Open chain)
Glucose – It is called Blood sugar or Grape sugar and occurs in 2-forms, i.e. D-form (Dextro-) and L-form (Levo-). All naturally occurring sugars are in D-form. It is an aldose sugar.
Fructose – It is called Fruit sugar. It is the most common sugar in plants. It is sweetest amongst naturally occurring sugars. It is a ketose sugar
Oligosaccharides
They contain 2 to 10 monosaccharide molecules.
A. Disaccharides – They contain 2-monosaccharides
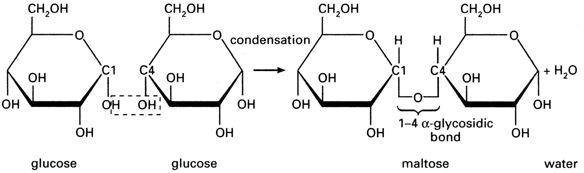
(i) Maltose – It is malt sugar and is formed during germination of starchy seeds. It is a reducing sugar. It contains 2 α – glucose units with α – 1,4 linkage/glycosidic bond
(ii) Lactose – It is milk sugar. It is also reducing sugar. It contains 1α-glucose and 1β-galactose (with β–1,4-linkage/glycosidic bond). Lactose is maximum in human milk. The galactose, produced from milk digestion, is also the constituent of AgarAgar.
(iii) Sucrose – It is the sugar of sugar cane and sugar beet. It is non-reducing sugar as it does not have free aldehyde or ketose groups. It contains 1 α-glucose and 1 β-fructose units. (α – 1- 2 linkage/glycosidic bond) The equimolecular mixture of glucose and fructose is called Invert Sugar which is sweeter than sucrose.
(iv) Trehalose – It is a disaccharide (α 1-1 linkage) present in micro-organisms. It is also a non-reducing sugar.
(v) Cellobiose – It contains 2 β-glucose units (β 1-4 linkage). It cannot be digested by mammalian enzymes.
B. Trisaccharides – They contain 3 monosaccharides, ex. Raffi nose – 1 glucose + 1
fructose + 1 galactose
C. Dextrin – It is also an oligosaccharide and is formed during starch-digestion.
Polysaccharides
- They are formed by joining of Monosaccharides (Monomers) by Glycosidic bonds between 1-4 carbon atoms. (In a polysaccharide chain the right end is called reducing end and the left end is called non-reducing end.
- They are non-reducing and mostly insoluble in water.
Two types – 1. Storage polysaccharides – e.g. Glycogen and Starch
2. Structural polysaccharides – e.g. Cellulose and Chitin
- Glycogen – It is present in animals (also called animal starch). It is a branched chain compound and has about 30 α-glucose units. It gives ‘red colour’ with an iodine solution.
- Starch – It is present in plants. The natural starch contains a mixture of amylose (10-20%) and amylopectin (80-90%), latter branched and insoluble in water. It also contains all α-glucose. (Amylose in starch is responsible for ‘deep blue colour’ with iodine)
- Cellulose–
- It contains all β-glucose.
- It is the most abundant organic compound in the biosphere.
- It is a fibrous polysaccharide and forms cell wall in plants.
- It forms roughage in human food.
- It is a straight chain (unbranched) compound.
- It forms 25 to 50% of wood and about 90% of cotton.
- Each molecule of cellulose contains about 6000 units of monosaccharides (glucose) and hence is Homoglycan hexosan.
- Rayon, an artifi cial and regenerated fi bre, is produced from cellulose.
4. Chitin – It forms exoskeleton, mainly in arthropods. It is also present in the cell wall of fungi. Its unit is β-N- Acetylglucosamine. It is a homopolymer. The polysaccharide Agar has more than one type of Monosaccharide units (hence Heteroglycan). The polysaccharide Inulin (Dahlia starch) is a polymer of fructose (Homoglycan) with β-1,2 linkage.
Conjugated or Complex Carbohydrates
They contain carbohydrate with non-carbohydrate units
- Glycoproteins – e.g., Blood antigens, Collagen, Lens protein, Blood protein, Hormones like FSH, LH, TSH, hCG, and in cell membranes.
- Glycolipids – e.g., Blood antigens, nerve fibres and cell membranes
- Mucopolysaccharides – e.g., Heparin. Hyaluronic acid, Synovial fluid, Vitrous humour, Cell wall in bacteria and Mucilages ( Galactose and mannose). They are also present in Bhindi (Lady’s finger) and Isabgol
Glucose Test – Benedict’s test, Fehling’s test.


Leave a Reply
You must be logged in to post a comment.